Jason Barker - Medical Cannabis Patient & Organizer with LECUA Patient’s Coalition Of New Mexico 
LECUAPatientsCoalitionNM@gmail.com
dukecitywellness.blogspot.com

Tuesday, February 28th 2017
New Mexico State Department of Health
Medical Cannabis Advisory Board
Medical Cannabis Program
PO Box 26110
Santa Fe, NM, 87502-6110
Medical Cannabis Program
PO Box 26110
Santa Fe, NM, 87502-6110
Petition: Requesting The Inclusion Of A New Medical Condition: Autism Spectrum Disorder
Table of Contents
Pg. 1 Cover Page
Pg. 2 - 3 Petition Introduction
Pg. 3 Petition Purpose and Background
Pg. 22 Relief Requested In Petition
Pg. (Noted) References
Pg. 22-23 Appendix A
Printing Provided By:
Petition Introduction: Inclusion Of A New Medical Condition: Autism Spectrum Disorder
New Mexico’s medical cannabis history started in 1978, after public hearings the legislature enacted H.B. 329, the nation’s first law recognizing the medical value of cannabis. The New Mexico’s medical cannabis program (MCP) is the only program in the U.S. that places sole responsibility for regulation on the state’s Department of Health. Doctors must comply with state requirements for patients to be considered for applying to the medical cannabis program.
In the Lynn and Erin Compassionate Use Act, (2007) the law states; The Secretary of Health shall establish an advisory board consisting of eight practitioners representing the fields of neurology, pain management, medical oncology, psychiatry, infectious disease, family medicine and gynecology. The practitioners shall be nationally board-certified in their area of specialty and knowledgeable about the medical use of cannabis. The members shall be chosen for appointment by the Secretary from a list proposed by the New Mexico Medical Society. A quorum of the advisory board shall consist of three members. The advisory board shall:
A. review and recommend to the department for approval additional debilitating medical conditions that would benefit from the medical use of cannabis;
B. accept and review petitions to add medical conditions, medical treatments or diseases to the list of debilitating medical conditions that qualify for the medical use of cannabis;
C. convene at least twice per year to conduct public hearings and to evaluate petitions, which shall be maintained as confidential personal health information, to add medical conditions, medical treatments or diseases to the list of debilitating medical conditions that qualify for the medical use of cannabis;
D. issue recommendations concerning rules to be promulgated for the issuance of the registry identification cards; and
E. recommend quantities of cannabis that are necessary to constitute an adequate supply for qualified patients and primary caregivers.
First, do no harm. As an important step in becoming a doctor, medical students must take the Hippocratic Oath. And one of the promises within that oath is “first, do no harm”.
A. review and recommend to the department for approval additional debilitating medical conditions that would benefit from the medical use of cannabis;
B. accept and review petitions to add medical conditions, medical treatments or diseases to the list of debilitating medical conditions that qualify for the medical use of cannabis;
C. convene at least twice per year to conduct public hearings and to evaluate petitions, which shall be maintained as confidential personal health information, to add medical conditions, medical treatments or diseases to the list of debilitating medical conditions that qualify for the medical use of cannabis;
D. issue recommendations concerning rules to be promulgated for the issuance of the registry identification cards; and
E. recommend quantities of cannabis that are necessary to constitute an adequate supply for qualified patients and primary caregivers.
First, do no harm. As an important step in becoming a doctor, medical students must take the Hippocratic Oath. And one of the promises within that oath is “first, do no harm”.
We have a sound law in the Lynn and Erin Compassionate Use Act, as Section 2 reads; PURPOSE OF ACT.--The purpose of the Lynn and Erin Compassionate Use Act is to allow the beneficial use of medical cannabis in a regulated system for alleviating symptoms caused by debilitating medical conditions and their medical treatments.
“ARTICLE 2B. LYNN AND ERIN COMPASSIONATE USE ACT
N.M. Stat. Ann. § 26-2B-2 (2009)
§ 26-2B-2. Purpose of act
The purpose of the Lynn and Erin Compassionate Use Act [26-2B-1 NMSA 1978] is to allow the beneficial use of medical cannabis in a regulated system for alleviating symptoms caused by debilitating medical conditions and their medical treatments.
HISTORY: Laws 2007, ch. 210, § 2.
EFFECTIVE DATES. --Laws 2007, ch. 210, § 12 makes the act effective July 1, 2007.”
Mosby’s Medical Dictionary states that “medical treatment” means; the management and care of a patient to combat disease or disorder. Medical treatment includes: Using prescription medications, or use of a non-prescription drug at prescription strength; and or treatment of disease by hygienic and pharmacologic remedies, as distinguished from invasive surgical procedures. Treatment may be pharmacologic, using drugs; surgical, involving operative procedures; or supportive, building the patient's strength. It may be specific for the disorder, or symptomatic to relieve symptoms without effecting a cure.(Mosby's Medical Dictionary, 9th edition.)
What is a chronic medical condition?
A chronic disease is one lasting 3 months or more, by the definition of the U.S. National Center for Health Statistics. Chronic diseases generally cannot be prevented by vaccines or cured by medication, nor do they just disappear. Harvard Medical Dictionary defines chronic as: Any condition that lasts a long time or recurs over time; chronic pain as: Pain that persists after an injury has healed or a disease is over; and chronic pain syndrome as : Long-term, severe pain that doesn't spring from an injury or illness, that interferes with daily life, and is often accompanied by other problems, such as depression, irritability, and anxiety.
A chronic disease is one lasting 3 months or more, by the definition of the U.S. National Center for Health Statistics. Chronic diseases generally cannot be prevented by vaccines or cured by medication, nor do they just disappear. Harvard Medical Dictionary defines chronic as: Any condition that lasts a long time or recurs over time; chronic pain as: Pain that persists after an injury has healed or a disease is over; and chronic pain syndrome as : Long-term, severe pain that doesn't spring from an injury or illness, that interferes with daily life, and is often accompanied by other problems, such as depression, irritability, and anxiety.
What is the meaning of debilitating?
Something that's debilitating seriously affects someone or something's strength or ability to carry on with regular activities, like a debilitating illness. Debilitating comes from the Latin word debilis, meaning "weak." That's why you'll often see the adjective used to describe illness, despite the negative reference.
Something that's debilitating seriously affects someone or something's strength or ability to carry on with regular activities, like a debilitating illness. Debilitating comes from the Latin word debilis, meaning "weak." That's why you'll often see the adjective used to describe illness, despite the negative reference.
Petition Purpose and Background
The purpose of this petition is; Inclusion Of A New Medical Condition: Autism Spectrum Disorder.
This petition for the Inclusion Of A New Medical Condition: Autism Spectrum Disorder is being provided to the state Department of Health Medical Cannabis Program so the advisory board can review and recommend to the department for approval additional debilitating medical conditions that would benefit from the medical use of cannabis with the Lynn and Erin Compassionate Use Act.
Who Should Qualify for Medical Cannabis Use?
According to Americans For Safe Access Policy Studies & Research:
Background: The most fundamental aspect of medical cannabis laws is the relationship between a patient and their physician. It is often only the physician and the patient that possess information about a patient’s health condition. However, many public officials and others who oppose medical cannabis laws often make assumptions about people’s health. The media have even fomented such inappropriate assumptions by naming a category of patients “Young Able Bodied Males,” condemning certain patients by visual assessment alone.
Findings: The health care information discussed between a patient and physician is considered private and protected under federal HIPAA laws. It is typically the purview of state medical boards to assess whether a physician has inappropriately recommended cannabis to someone who should not be qualified. Studies have shown in some medical cannabis states that the majority of patients suffer from chronic pain, an ailment that is not obviously detectable by another person. Nevertheless, police will often harass and arrest patients based on the assumption that someone is faking their illness.
Position: Medical professionals should have an unrestricted ability to recommend cannabis therapeutics and that should not be impacted by law enforcement’s perceptions.
According to Americans For Safe Access Policy Studies & Research:
Background: The most fundamental aspect of medical cannabis laws is the relationship between a patient and their physician. It is often only the physician and the patient that possess information about a patient’s health condition. However, many public officials and others who oppose medical cannabis laws often make assumptions about people’s health. The media have even fomented such inappropriate assumptions by naming a category of patients “Young Able Bodied Males,” condemning certain patients by visual assessment alone.
Findings: The health care information discussed between a patient and physician is considered private and protected under federal HIPAA laws. It is typically the purview of state medical boards to assess whether a physician has inappropriately recommended cannabis to someone who should not be qualified. Studies have shown in some medical cannabis states that the majority of patients suffer from chronic pain, an ailment that is not obviously detectable by another person. Nevertheless, police will often harass and arrest patients based on the assumption that someone is faking their illness.
Position: Medical professionals should have an unrestricted ability to recommend cannabis therapeutics and that should not be impacted by law enforcement’s perceptions.
“Qualifying medical condition” shall mean any condition for which treatment with medical cannabis would be beneficial, as determined by a patient's qualified medical professional, including but not limited to cancer, glaucoma, positive status for human immunodeficiency virus, acquired immune deficiency syndrome (AIDS), hepatitis C, amyotrophic lateral sclerosis (ALS), Crohn’s disease, Parkinson’s disease, post-traumatic stress disorder, arthritis, chronic pain, neuropathic and other intractable chronic pain, and multiple sclerosis.
“Qualifying patient” shall mean a person who has a written recommendation from a qualified medical professional for the medical use of cannabis.
Part One: The Endocannabinoid System and Autism Spectrum Disorder (ASD)
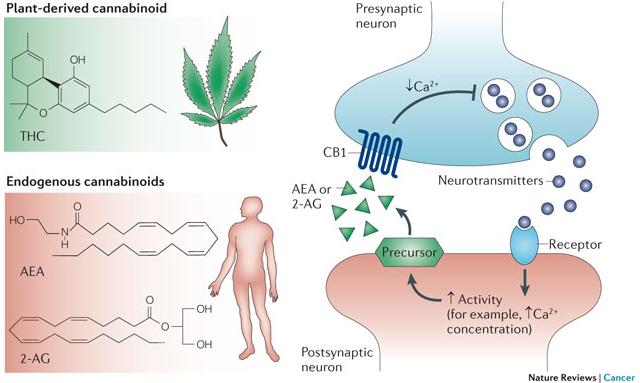
The importance of the discovery of the role that the endocannabinoid system (ECS) plays in human health and disease cannot be understated. Cannabinoid receptors are the most highly expressed of any G-protein coupled receptor (GPCR) in the body. They’re the only ones to play a direct role in virtually every aspect of the human body (CNS and immune systems, throughout the periphery, presynaptic, and postsynaptic).[1]
It’s no wonder that anecdotal reports of cannabis treatments indicate effectiveness in such a wide array of conditions. The growing body of scientific research surrounding the endocannabinoid system continues to lead to the further understanding of the physiological basis in a growing number of conditions.[45]
One condition with both supportive anecdotal and preclinical scientific evidence is for patients on the severe end of the autism spectrum (ASD). In a short series of articles we’ll attempt to shed light on the role that the endocannabinoid system plays in the progression of autism, the potential role of phytocannabinoids in treatment, and what that might mean in a practical sense.
NL3 Mutations Inhibit Tonic Endocannabinoid Secretions
Neuroligins are part of a family of neuronal cell surface proteins that “connect presynaptic and postsynaptic neurons at synapses, mediate signaling across the synapse, and shape the properties of neural networks by specifying synaptic functions”. Alterations in genes encoding neuroligins are associated with autism and other cognitive diseases.[56]
Mutations in neuroligin-3 (NL3), a member of the family of neuroligins, are associated with ASD.[17] NL3 is required for tonic secretion of endocannabinoids (AEA, 2-AG).[17] NL3 mutations have been shown to inhibit tonic endocannabinoid secretion.[17] This dysregulation in endocannabinoid signaling may contribute to the pathophysiology of autism.[17, 50, 53] These findings have in part prompted researchers to apply to conduct research with nonhuman primates in order to further elucidate this association.[39]
Targeting Endocannabinoid System to Treat FXS
Fragile X syndrome (FXS) is the most commonly known genetic cause of autism.[30] FXS is associated with a loss of the fragile X mental retardation protein (FMRP) which regulates signal transduction in the brain.[30] This FMRP deficiency is believed to “increase neuronal excitability which is mediated by endocannabinoids”.[59]
FXS is also associated with “neuropsychiatric problems such as hyperactivity, attention disorders, and seizures.”[19] The endocannabinoid system is key to modulating functions that are involved with regulating all of these disorders including “synaptic plasticity, cognitive performance, anxiety, nociception and seizure susceptibility.”[19] The endocannabinoid system is specifically implicated in just about all aspects of FXS including “behavioral, synaptic and molecular manifestations.”[19] Preclinical research implicates CB1 and CB2 as pharmacological targets with the potential to reduce cognitive deficits and anxiety in FXS models in rodents.[19, 59]
Increased Expression of CB2 Receptors Associated with ASD
Though it wasn’t long ago that the role that CB2 receptors played in the human brain was believed to be negligible, additional research has implicated it as having a much more substantial role than previously understood.
“Given that CB2 is up-regulated, and that it’s believed to play a neuroprotective role, CB2 is being investigated as a potential target for treatment of ASD.[53]”
One example is that CB2 is believed to play a neuroprotective role in response to a variety of inflammatory stimuli, this has implications in a number of neuropsychiatric conditions including ASD.[4, 16, 53]
In ASD, as well as a number of conditions, the expression level of CB2 receptors increases in response to the inflammatory nature of the condition.[16, 53] Given that CB2 is up-regulated, and that it’s believed to play a neuroprotective role, CB2 is being investigated as a potential target for treatment of ASD.[53]
Elevated Cytokine Levels Associated with ASD
“Cytokines are small secreted proteins released by cells that have a specific effect on the interactions and communications between cells… Pro-inflammatory cytokines are involved in the up-regulation of inflammatory reactions.”[60]
Elevated pro-inflammatory cytokine levels are associated with ASD.[44] Whether this is due in part as a result of NL3 mutations inhibiting tonic secretion of endocannabinoids remains uncertain. However, endocannabinoids (AEA, 2-AG) have been shown to play key roles inhibiting cytokines via CB2.[12, 47]
The majority of cannabinoids have been demonstrated to decrease cytokine production via CB1/CB2 dependent and independent mechanisms.[25, 27, 29, 36]
Clinically Diagnosing ASD via the ECS
A team of researchers recently discovered and patented a process that claims that it’s possible to clinically diagnose ASD, and susceptibility to it, via observation of the degree of modulation that acetaminophen has on endocannabinoid levels. However, based on a series of deductions made within their published literature, it appears that additional research is required.
Other Relevant ECS/ASD Implications
The number of functions that ECS regulate is extensive and beyond the scope of this paper.[45, 48] However, a few potentially relevant aspects to ASD will be listed:
- “CB1 variations modulate the striatal function that underlies the perception of signals of social reward, such as happy faces. This suggests that CB1 is a key element in the molecular architecture of perception of certain basic emotions. This may have implications for understanding neurodevelopmental conditions marked by atypical eye contact and facial emotion processing, such as ASC.”[13]
- Additional targets of endocannabinoids (and exogenous cannabinoids), PPARα, PPARγ, and GPR55 expression levels have shown reductions in a valproic acid model of autism in rats.[33]
Conclusion
Based on the preclinical research the endocannabinoid system appears to be directly impacted by, as well as a potential target for treatment of, physiological manifestations of genetic factors associated with ASD including NL3 mutations and FXS. NL3 mutations inhibit tonic secretion of endocannabinoids and disrupt their signaling. This possibly contributes to the identified increase in proinflammatory cytokines levels in ASD. CB2 is upregulated in the brain in response to inflammatory stimuli as part of a neuroprotective role, and is suggested as a target for treatment. There appears to be a preponderance of evidence that the ECS is involved in the progression of ASD.
Citations & References
- Alger, Bradley. "Getting High on the Endocannabinoid System." Cerebrum (2013).
- Andó, Rómeó D., et al. "The inhibitory action of exo-and endocannabinoids on [3H] GABA release are mediated by both CB1 and CB2 receptors in the mouse hippocampus." Neurochemistry International 60.2 (2012): 145-152.
- Aso, Ester, et al. "Lack of CB1 receptor activity impairs serotonergic negative feedback." Journal of neurochemistry 109.3 (2009): 935-944.
- Benito, C., et al. "Cannabinoid CB2 receptors in human brain inflammation." British journal of pharmacology 153.2 (2008): 277-285.
- Best, Aaron R., and Wade G. Regehr. "Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses." The Journal of Neuroscience 28.25 (2008): 6508-6515.
- Bolognini, D., et al. "Cannabidiolic acid prevents vomiting in Suncus murinus and nausea‐induced behaviour in rats by enhancing 5‐HT1A receptor activation." British journal of pharmacology 168.6 (2013): 1456-1470.
- Booz, George W. "Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress." Free Radical Biology and Medicine 51.5 (2011): 1054-1061.
- Braida, Daniela, et al. "5-HT1A receptors are involved in the anxiolytic effect of ∆9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague–Dawley rats." European journal of pharmacology 555.2 (2007): 156-163.
- Carey, Megan R., et al. "Presynaptic CB1 receptors regulate synaptic plasticity at cerebellar parallel fiber synapses." Journal of neurophysiology 105.2 (2011): 958.
- Carley, David W., et al. "Functional role for cannabinoids in respiratory stability during sleep." Sleep 25.4 (2002): 391-398.
- Castillo, Pablo E., et al. "Endocannabinoid signaling and synaptic function." Neuron 76.1 (2012): 70-81.
- Cencioni, Maria Teresa, et al. "Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors." PLoS One 5.1 (2010): e8688.
- Chakrabarti, Bhismadev, and Simon Baron-Cohen. "Variation in the human Cannabinoid Receptor (CNR1) gene modulates gaze duration for happy faces." Molecular autism 2.1 (2011): 10.
- Di Filippo, Clara, et al. "Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: involvement of cytokine/chemokines and PMN." Journal of leukocyte biology 75.3 (2004): 453-459.
- Di Marzo, V., and F. Piscitelli. "Gut feelings about the endocannabinoid system." Neurogastroenterology & Motility 23.5 (2011): 391-398.
- Fernández‐Ruiz, Javier, et al. "Prospects for cannabinoid therapies in basal ganglia disorders." British journal of pharmacology 163.7 (2011): 1365-1378.
- Földy, Csaba, Robert C. Malenka, and Thomas C. Südhof. "Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling." Neuron 78.3 (2013): 498-509.
- Gamage, Thomas F., and Aron H. Lichtman. "The endocannabinoid system: role in energy regulation." Pediatric blood & cancer 58.1 (2012): 144-148.
- Garcia, Arnau Busquets, et al. “New insights into the molecular pathophysiology of fragile X syndrome and therapeutic perspectives from the animal model”, International Journal of Biochemistry and Cell Biology, 53 (2014) 121-126.
- Godlewski, Grzegorz, Manfred Göthert, and Barbara Malinowska. "Cannabinoid receptor‐independent inhibition by cannabinoid agonists of the peripheral 5‐HT3 receptor‐mediated von Bezold–Jarisch reflex." British journal of pharmacology 138.5 (2003): 767-774.
- Gomes, Felipe V., Leonardo BM Resstel, and Francisco S. Guimarães. "The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors." Psychopharmacology 213.2-3 (2011): 465-473.
- Gong, Jian-Ping, et al. "Cannabinoid CB2 receptors: immunohistochemical localization in rat brain." Brain research 1071.1 (2006): 10-23.
- Haj-Dahmane, Samir, and Roh-Yu Shen. "Modulation of the serotonin system by endocannabinoid signaling." Neuropharmacology 61.3 (2011): 414-420.
- Iring, András, et al. "Role of Endocannabinoids and Cannabinoid-1 Receptors in Cerebrocortical Blood Flow Regulation." PloS one 8.1 (2013): e53390.
- Izzo, Angelo A., et al. "Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb." Trends in pharmacological sciences 30.10 (2009): 515-527.
- Jean-Gilles, Lucie, Bruno Gran, and Cris S. Constantinescu. "Interaction between cytokines, cannabinoids and the nervous system." Immunobiology 215.8 (2010): 606-610.
- Jiang, Chengyu, Adrian T. Ting, and Brian Seed. "PPAR-γ agonists inhibit production of monocyte inflammatory cytokines." Nature 391.6662 (1998): 8286.
- Johnson, Jeremy R., et al. "Multicenter, double-blind, randomized, placebocontrolled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain." Journal of pain and symptom management 39.2 (2010): 167-179.
- Juknat, Ana, et al. "Cannabidiol affects the expression of genes involved in zinc homeostasis in BV-2 microglial cells." Neurochemistry international 61.6 (2012): 923-930.
- Jung, Kwang-Mook, et al. "Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome." Nature communications 3 (2012): 1080.
- Katona, István, and Tamás F. Freund. "Multiple functions of endocannabinoid signaling in the brain." Annual review of neuroscience 35 (2012): 529-558.
- Kawamura, Yoshinobu, et al. "The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum." The Journal of neuroscience 26.11 (2006): 2991-3001.
- Kerr, D. M., et al. "Alterations in the endocannabinoid system in the rat valproic acid model of autism." Behavioural brain research 249 (2013): 124-132.
- Kishimoto, Yasushi, and Masanobu Kano. "Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning." The Journal of neuroscience 26.34 (2006): 8829-8837.
- Klegeris, Andis, Christopher J. Bissonnette, and Patrick L. McGeer. "Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid‐type CB2 receptor." British journal of pharmacology 139.4 (2003): 775-786.
- Kozela, Ewa, et al. "Cannabinoids ∆9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferonβ/STAT proinflammatory pathways in BV-2 microglial cells." Journal of biological chemistry 285.3 (2010): 1616-1626.
- Li, Chen, Peter M. Jones, and Shanta J. Persaud. "Role of the endocannabinoid system in food intake, energy homeostasis and regulation of the endocrine pancreas." Pharmacology & therapeutics 129.3 (2011): 307-320.
- Machado Bergamaschi, Mateus, et al. "Safety and side effects of cannabidiol, a Cannabis sativa constituent." Current drug safety 6.4 (2011): 237-249.
- Malcher-Lopes, Renato. "Targeting alterations in the endocannabinoid system of rodents and non-human primates for the study of autism." Qatar Foundation Annual Research Conference. No. 2013. 2013.
- Marco, Eva M., et al. "Endocannabinoid system and psychiatry: in search of a neurobiological basis for detrimental and potential therapeutic effects." Frontiers in behavioral neuroscience 5 (2011).
- Mato, Susana, et al. "CB1 knockout mice display impaired functionality of 5‐HT1A and 5‐HT2A/C receptors." Journal of neurochemistry 103.5 (2007): 2111-2120.
- Mikics, Eva, et al. "Interactions between the anxiogenic effects of CB1 gene disruption and 5-HT3 neurotransmission." Behavioural pharmacology 20.3 (2009): 265-272.
- Müller‐Vahl, K. R., et al. "Cannabinoids: possible role in patho‐physiology and therapy of Gilles de la Tourette syndrome." Acta Psychiatrica Scandinavica 98.6 (1998): 502-506.
- Napolioni, Valerio, et al. "Plasma cytokine profiling in sibling pairs discordant for autism spectrum disorder." Journal of neuroinflammation 10.1 (2013): 38.
- Pacher, Pál, Sándor Bátkai, and George Kunos. "The endocannabinoid system as an emerging target of pharmacotherapy." Pharmacological reviews 58.3 (2006): 389-462.
- Palazuelos, Javier, et al. "CB2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling." Journal of Biological Chemistry 287.2 (2012): 1198-1209.
- Panikashvili, David, et al. "The endocannabinoid 2-AG protects the blood–brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines." Neurobiology of disease 22.2 (2006): 257-264.
- Pertwee, R. G., et al. "International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2." Pharmacological reviews 62.4 (2010): 588-631.
- Pomerantz, Daniel J. "THE ROLE OF CB2 ENDOCANNABINOID RECEPTOR AND MTORC1 IN NEUROPROGENITOR CELL PROLIFERATION IN TUBEROUS SCLEROSIS." Emphasis Program (2013): 73.
- Onaivi, E. S., et al. "Consequences of cannabinoid and monoaminergic system disruption in a mouse model of autism spectrum disorders." Current neuropharmacology 9.1 (2011): 209.
- Rock, Erin. Cannabidiol Indirectly Activates 5-HT1A Somatodendritic Autoreceptors to Attenuate Vomiting and Nausea. Diss. 2011.
- Roloff, Alan M., et al. "Homer 1a gates the induction mechanism for endocannabinoid-mediated synaptic plasticity." The Journal of Neuroscience 30.8 (2010): 3072-3081.
- Siniscalco, Dario, et al. "Cannabinoid receptor type 2, but not type 1, is upregulated in peripheral blood mononuclear cells of children affected by autistic disorders." Journal of autism and developmental disorders 43.11 (2013): 26862695.
- Sharkey, Keith A., Nissar A. Darmani, and Linda A. Parker. "Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system." European journal of pharmacology 722 (2014): 134-146.
- Stone, Joe, et al. “Cannabinoids, Ketogenic Diets, Holy Basil, and the PPAR Connection.” Unpublished (2014) http://www.scribd.com/doc/207827158/Cannabinoids-Ketogenic-Diets-HolyBasil-and-the-PPAR-Connection
- Sudhof, Thomas C., et al. “Neuroligins and neurexins link synaptic function to cognitive disease”, Nature, 2008/10/16/print, Nature Publishing Group, http://dx.doi.org/10.1038/nature07456
- Tanimura, Asami, et al. "Not glutamate but endocannabinoids mediate retrograde suppression of cerebellar parallel fiber to Purkinje cell synaptic transmission in young adult rodents." Neuropharmacology 57.2 (2009): 157-163.
- Vaney, C., et al. "Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study." Multiple Sclerosis 10.4 (2004): 417-424.
- Zhang Longhua, Alger Bradley E., et al. “Enhanced Endocannabinoid Signaling Elevates Neuronal Excitability in Fragile X Syndrome” 2010
- Zhang, Jun-Ming, and Jianxiong An., et al. “Cytokines, Inflammation and Pain.” International anesthesiology clinics2 (2007): 27–37. PMC. Web. 11 June 2015.
Part Two: The Role of Phytocannabinoids in ASD Therapy
In part one of this series the preclinical scientific evidence illustrating the involvement of the endocannabinoid system (ECS) in the physiological progression of Autism Spectrum Disorder (ASD) was provided. Here we’ll highlight some of the pharmacological characteristics that phytocannabinoids share with endocannabinoids, the use of phytocannabinoids in the treatment of symptoms and diseases associated with ASD, and why having access to a variety of cannabis chemotypes will always be preferable to a select cannabinoid and/or ratio.
The anecdotal reports of successful cannabinoid therapies seem to be supported by the fact that phytocannabinoids from cannabis, and other natural sources, display similar pharmacological characteristics to that of endocannabinoids that are dysregulated in ASD.[31] The potential therapeutic value of supplementing the endocannabinoid system with phytocannabinoids has been suggested in the treatment of a number of diseases with suspected underlying endocannabinoid deficiencies.[34] Documentation of the safety and clinical efficacy of phytocannabinoids in a variety of treatments continues to grow.[16] In regards to the treatment of ASD, some similar characteristics are worth highlighting, including:
Based on preclinical research ECS deficiencies appear to be associated with ASD, and it’s implicated as a potential target for treatment. Phytocannabinoids target the ECS and display similar pharmacological characteristics to endocannabinoids that are dysregulated. It’s been suggested that therapies for conditions with corresponding ECS deficiencies may include supplementation with phytocannabinoids. This seems to potentially support the anecdotal reports of successful cannabinoid therapies in ASD.
Treating Symptoms Associated with ASD
There is a considerable body of supportive preclinical data in regards to targeting the ECS with phytocannabinoids in the treatment of a number of symptoms and diseases associated with ASD. For sake of brevity some of these will be highlighted and cited:
Again, anecdotal reports of success appear to be supported by an abundance of preclinical research that indicates a potential role for phytocannabinoids in the treatment of symptoms and diseases associated with ASD.
Botanical Extracts > Dronabinol
Due to the relatively common off-label use of Dronabinol (a man-made/synthetic form of THC), for ASD therapy, it seems relevant to point out the substantial data, including clinical studies, suggesting that the combined administration of CBD along with THC (and possibly other cannabinoids/terpenes present in cannabis) exhibit additive and synergistic effects. This is known as the entourage effect and results in greater clinical efficacies when compared to either cannabinoid alone.[25, 18, 34]
The second most prominent cannabinoid in cannabis is cannabidiol (CBD).[13] CBD has been shown to inhibit intoxication, sedation, and tachycardia associated with delta-9-tetrahydrocannabinol (THC).[34] It’s been shown to increase the clinical efficacy of THC, while adding therapeutic value in its own right.[34]
A large portion of the research conducted thus far with ASD and cannabinoids has been with Dronabinol (a synthetic form of THC) alone. Dronabinol has indicated potential for treatment in a single adolescent case study of autism.[22] Does that mean THC along with CBD might offer increased clinical efficacy similar to the way they have been demonstrated to with other conditions?[34] Based on the results of previous research and anecdotal reports this might be the case.
The added benefit of additional cannabinoids (and the added benefit of specifically tailoring ratios) is an important component that sets botanical extracts from cannabis apart from THC or CBD alone. This is why having access to a variety of cannabis chemotypes will always have more potential for therapeutic value than a select cannabinoid, ratio, or cannabis chemotype.
Discussion
Based on their ability to target the ECS, and their shared characteristics with dysfunctional endocannabinoid levels, preclinical evidence supports the potential therapeutic value of phytocannabinoids in ASD therapy.
Highlighting individual pharmacological characteristics of CBD, THC, and other phytocannabinoids is beyond the scope of this paper. However, based on the ECS deficiencies associated with ASD, and the ability of phytocannabinoids to target and modulate aspects of the deficiencies, anecdotal reports seem to be supported by the best available scientific data. It appears that phytocannabinoids have the potential for therapeutic value in some severe cases of ASD.
Citations & References
- Almeida, Valeria, et al. "Cannabidiol exhibits anxiolytic but not antipsychotic property evaluated in the social interaction test." Progress in NeuroPsychopharmacology and Biological Psychiatry 41 (2013): 30-35.
- Avraham, Hava Karsenty, et al. "The cannabinoid CB2 receptor agonist AM1241 enhances neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis." British journal of pharmacology2 (2014): 468-479.
- Borges, Rosivaldo S., et al. "Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants." Molecules10 (2013): 12663-12674.
- Camilleri, Michael, et al. "Cannabinoid receptor 1 gene and irritable bowel syndrome: phenotype and quantitative traits." American Journal of Physiology Gastrointestinal and Liver Physiology 304.5 (2013): G553-G560.
- Campos, Alline Cristina, et al. "Multiple mechanisms involved in the largespectrum therapeutic potential of cannabidiol in psychiatric disorders." Philosophical Transactions of the Royal Society B: Biological Sciences 367.1607 (2012): 3364-3378.
- Casarotto, Plinio C., et al. "Cannabidiol inhibitory effect on marble-burying behaviour: involvement of CB1 receptors." Behavioural pharmacology 21.4 (2010): 353-358.
- Choi, In-Young, et al. "Activation of Cannabinoid CB2 Receptor–Mediated AMPK/CREB Pathway Reduces Cerebral Ischemic Injury." The American journal of pathology 182.3 (2013): 928-939.
- Davis, Mellar, et al. "The emerging role of cannabinoid neuromodulators in symptom management." Supportive care in cancer1 (2007): 63-71.
- Deiana, Serena, et al. "Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), ∆9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour." Psychopharmacology 219.3 (2012): 859-873.
- Di Sabatino, A., et al. "The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease." Mucosal immunology 4.5 (2011): 574-583.
- Engeli, Stefan. "Central and peripheral cannabinoid receptors as therapeutic targets in the control of food intake and body weight." Appetite Control. Springer Berlin Heidelberg, 2012. 357-381.
- Garcia-Bonilla, Lidia, et al. "Immune mechanisms in cerebral ischemic tolerance." Frontiers in neuroscience 8 (2014).
- Gertsch, Jürg, Roger G. Pertwee, and Vincenzo Di Marzo. "Phytocannabinoids beyond the Cannabis plant–do they exist?." British journal of pharmacology 160.3 (2010): 523-529.
- Gomes, Felipe V., et al. "Facilitation of CB1 receptor-mediated neurotransmission decreases marble burying behavior in mice." Progress in NeuroPsychopharmacology and Biological Psychiatry 35.2 (2011): 434-438.
- Hampson, Aidan J., Julius Axelrod, and Maurizio Grimaldi. "Cannabinoids as antioxidants and neuroprotectants." U.S. Patent No. 6,630,507. 7 Oct. 2003.
- Hazekamp, Arno, and Franjo Grotenhermen. "Clinical Studies With Cannabis and Cannabinoids, 2005-2009." (2013).
- Hill, Matthew N., and Boris B. Gorzalka. "The endocannabinoid system and the treatment of mood and anxiety disorders." CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 8.6 (2009): 451-458.
- Izzo, Angelo A., et al. "Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb." Trends in pharmacological sciences 30.10 (2009): 515-527.
- Jiang, Wen, et al. "Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic-and antidepressant-like effects." Journal of Clinical Investigation11 (2005): 3104.
- Jones, Nicholas A., et al. "Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures." Seizure 21.5 (2012): 344-352.
- Krueger, Dilja D., and Nils Brose. "Evidence for a common endocannabinoidrelated pathomechanism in autism spectrum disorders." Neuron 78.3 (2013): 408410.
- Kurz, René, and Kurt Blaas. "Use of dronabinol (delta-9-THC) in autism: A prospective single-case-study with an early infantile autistic child." (2010)
- Lara-Celador, I. et al. “Using the Endocannabinoid System as a Neuroprotective Strategy in Perinatal Hypoxic-Ischemic Brain Injury.” Neural Regeneration Research8 (2013): 731–744.
- Marco, Eva M., et al. "The role of the endocannabinoid system in eating disorders: pharmacological implications." Behavioural pharmacology 23.5 and 6 (2012): 526-536.
- McPartland, John M., and Ethan B. Russo. "Cannabis and cannabis extracts: greater than the sum of their parts?." Journal of Cannabis Therapeutics 1.3-4 (2001): 103-132.
- Murikinati, Sasidhar, et al. "Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment." The FASEB journal 24.3 (2010): 788-798.
- Müller-Vahl, K. R., et al. "Cannabis in movement disorders." Forschende Komplementärmedizin/Research in Complementary Medicine 6.Suppl. 3 (2004): 23-27.
- Murillo-Rodriguez, Eric, et al. "The emerging role of the endocannabinoid system in the sleep-wake cycle modulation." Central Nervous System Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Central Nervous System Agents) 11.3 (2011): 189-196.
- Onaivi, E. S., et al. "Consequences of cannabinoid and monoaminergic system disruption in a mouse model of autism spectrum disorders." Current neuropharmacology 9.1 (2011): 209.
- Passie, Torsten, et al. "Mitigation of post‐traumatic stress symptoms by Cannabis resin: A review of the clinical and neurobiological evidence." Drug testing and analysis7-8 (2012): 649-659.
- Pertwee, R. G., et al. "International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2." Pharmacological reviews 62.4 (2010): 588-631.
- Porter, Brenda E., and Catherine Jacobson. "Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy." Epilepsy & Behavior 29.3 (2013): 574-577.
- Russo, Ethan, and Geoffrey W. Guy. "A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol." Medical hypotheses 66.2 (2006): 234-246.
- Russo, Ethan B. "Taming THC: potential cannabis synergy and phytocannabinoid‐terpenoid entourage effects." British Journal of Pharmacology 163.7 (2011): 1344-1364.
- Sanchez, A. J., and A. Garcia-Merino. "Neuroprotective agents: cannabinoids." Clinical Immunology1 (2012): 57-67.
- Schier, Alexandre Rafael de Mello, et al. "Cannabidiol, a Cannabis sativa constituent, as an anxiolytic drug." Revista Brasileira de Psiquiatria 34 (2012): 104-110.
- Schmidt, W., et al. "Cannabinoid receptor subtypes 1 and 2 mediate long-lasting neuroprotection and improve motor behavior deficits after transient focal cerebral ischemia." Neuroscience 227 (2012): 313-326.
- Shu, Hai-Feng, et al. "Expression of TRPV1 in cortical lesions from patients with tuberous sclerosis complex and focal cortical dysplasia type IIb." Brain and Development 35.3 (2013): 252-260.
- Russo, Ethan B. "Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions?." Neuro endocrinology letters 25.1-2 (2003): 31-39.
- van Rijn, Clementina M., et al. "Endocannabinoid system protects against cryptogenic seizures." Pharmacol Rep 63 (2011): 165-168.
- Ware, Mark A., et al. "The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial." Anesthesia & Analgesia 110.2 (2010): 604-610.
- Wright, K. L., M. Duncan, and K. A. Sharkey. "Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation." British journal of pharmacology 153.2 (2008): 263-270
- Youssef, F. F., and A. J. Irving. "From cannabis to the endocannabinoid system: refocussing attention on potential clinical benefits." West Indian Medical Journal 61.3 (2012).
- Zurolo, E., et al. "CB1 and CB2 cannabinoid receptor expression during development and in epileptogenic developmental pathologies." Neuroscience 170.1 (2010): 28-41.
Part Three: Practical Approach to Cannabis Based ASD Therapies
In our previous installment, we provided a brief overview of the preclinical research implicating the role that the endocannabinoid system (ECS) plays in the progression of autism spectrum disorder (ASD), preclinical research supporting targeting the ECS to treat ASD, and provided a number of preclinical studies indicating the potential value of phytocannabinoids in treating symptoms and diseases associated with ASD.
Here we’ll examine the shortcomings of current research, explore possible adverse effects of cannabinoid treatments, discuss the types of autism that may currently warrant cannabinoid treatments, and illustrate how a family might systematically approach a cannabinoid treatment for ASD.
Cannabis Therapy Uncertainties
While the preclinical research appears promising, it’s important to note that preclinical research doesn’t always translate into clinical efficacy; although, anecdotal reports of success do lend themselves to the idea of promise. Additionally, treatments are made more complex by the wide spectrum of ASD and the genetic conditions associated with it.[1, 2] Specific cannabinoids and ratios of cannabinoids (as well as terpenes and flavonoids) that offer the most therapeutic value may vary on an individual case by case basis.
Preclinical research does not yet provide clear cut answers in regards to how to target the endocannabinoid system to treat ASD. CB1 and CB2 blockade decreased cognitive deficits and anxiety, respectively, in some FXS models.[2, 10] Other models indicate the beneficial aspect of combating neuroinflammation via CB2 activation.[9, 5] Many of the symptoms associated with ASD are treated via CB1 activation as cited in the previous article of this installment. The NL3 models indicate a benefit from increasing endocannabinoid levels.[1] NL3 mutations and FXS both have a wide spectrum of dysfunction, the degree of which is different in every patient. [1, 2] Therefore, each patient may display varying ECS dysfunctions and, in turn, require varying ratios of cannabinoids for therapy. This is why it may be important to have access to a selection of cannabis varieties with known ratios of cannabinoids present (primarily CBD:THC).
Possible Adverse Effects
When considering a cannabis treatment for an underage patient and/or a developing brain there may be adverse effects from cannabis treatments that should be considered. Acute and chronic administration of delta-9-tetrahydrocannabinol (THC) has been demonstrated to cause mild cognitive deficits related to memory and learning via CB1 activation in healthy brains in some mouse models.[8]
“According to anecdotal reports, an increased ratio of THC is required to increase the efficacy of some treatments. The range seems to vary significantly from 24:1 to 1:1 (CBD:THC) with fewer outlying cases reporting success from higher THC ratios.”
While this characteristic is not to be downplayed or overlooked, it should be pointed out that low doses of THC also activate preconditioning and postconditioning mechanisms that protect the brain from more severe insults.[8] This may be relevant because autistic brains are more vulnerable to environmental toxicity, oxidative stress, neuroinflammation, and neuronal insults.[4] That being said the possibility of adverse effects from cannabinoid therapies should be considered in pediatric patients. This is due to the concern of the unknown impact that cannabis treatments, particularly THC, has on a developing brain.
An argument could be made that botanical extracts with cannabidiol (CBD) present might offer safer options for patients, with greater clinical efficacy, when compared to THC alone.[7] This is partly why having access to CBD is important to ASD therapies, but it offers much more therapeutically than simply increasing the safety and efficacy of THC.[3]
When is Cannabis Therapy the Right Choice?
If a patient has a form of ASD that is truly severe, is unresponsive to available treatments, and the patient’s condition will deteriorate if no therapy is implemented, we believe that their family and physician should be legally permitted to make the decision as to whether botanical extracts from cannabis may be a viable option for treatment. Additionally, having access to both CBD and THC is optimal for the success of a cannabis therapy for ASD.
A Practical Approach to Cannabis Therapy
How can combinations of cannabinoids be put into practical use by individual families? For our purposes let’s review the anecdotal reports of cannabinoid based treatments currently being utilized in ASD as well as pediatric epilepsy. This might be a good comparison due to the range and complexity of both conditions and similarities in genetic dysfunctions. CBD-only extracts continue to prove effectiveness in treating many types of epilepsy, but not all.[6] According to anecdotal reports, an increased ratio of THC is required to increase the efficacy of some treatments. The range seems to vary significantly from 24:1 to 1:1 (CBD:THC) with fewer outlying cases reporting success from higher THC ratios.
Due to the range of ASD it seems possible that, similar to cannabinoid-based epilepsy treatments, varying ratios of cannabinoids (specifically CBD:THC) will prove to have a greater efficacy overall when compared to individual cannabinoid based treatments. The greater the ability to adjust the cannabinoid ratios the more optimal the conditions are to achieve therapeutic value. Though, generally speaking, they’re predominantly extracts that are high in CBD content.
“In ASD treatments, a first line of action to approaching a cannabis therapy might be to try a particularly high CBD ratio.”
In ASD treatments, a first line of action to approaching a cannabis therapy might be to try a particularly high CBD ratio. A high CBD containing botanical extract would be taken orally. The quantity of this botanical extract would slowly be increased incrementally based on weight until a desired effect, no effect, or an adverse effect is identified. If CBD alone is not sufficient to achieve a desired effect, a strain with higher content of THC is used, or an additional botanical extract that has a high THC content is added to a CBD extract in order to achieve the desired CBD:THC ratio. Again, this is incrementally increased based on weight until a desired effect, no effect, or an adverse effect is identified. More information on regiments can be found on Facebook in groups focusing on cannabis based pediatric therapies.
The ability to procure various ratios of cannabinoids in order to specifically tailor therapies may prove paramount to the effectiveness of treatments. Generally when parents find a strain or ratio that works well for them they try and stick with it. There are numerous cannabis varieties with varying ratios of cannabinoids in the majority of medical cannabis States. Seeking out plants that have been used in other ASD or epilepsy treatments might be a good first step.
Moving Forward
As future research is published we may see advances in the treatment of ASD by targeting the ECS. Until then we remain largely in the dark with possible glimmers of hope on the horizon. The mere possibility of combating ASD is enough for some families to explore cannabis as a treatment option, generally families unable to wait for possible future advances.
The question of when it becomes acceptable to provide a cannabis based treatment to a pediatric patient is complicated and loaded with moral and legal implications. The wide spectrum of ASD, the uncertainties in regards to targeting the endocannabinoid system for this treatment, and the possible adverse effects of THC therapy in pediatrics, all lend to the complexity of the issue.
In States with approved medical cannabis programs, it’s imperative that ASD be added to their lists of qualified medical conditions for approval of cannabis licenses. Parents and physicians should have the right and legal protection to explore cannabis as a treatment option, especially as a last-line therapy in ASD.
Citations & References
- Földy, Csaba, Robert C. Malenka, and Thomas C. Südhof. "Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling." Neuron 78.3 (2013): 498-509.
- Garcia, Arnau Busquets, et al. “New insights into the molecular pathophysiology of fragile X syndrome and therapeutic perspectives from the animal model”, International Journal of Biochemistry and Cell Biology, 53 (2014) 121-126.
- Izzo, Angelo A., et al. "Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb." Trends in pharmacological sciences 30.10 (2009): 515-527.
- Kern, Janet K., and Anne M. Jones. "Evidence of toxicity, oxidative stress, and neuronal insult in autism." Journal of Toxicology and Environmental Health, Part B 9.6 (2006): 485-499
- Malcher-Lopes, Renato. "Targeting alterations in the endocannabinoid system of rodents and non-human primates for the study of autism." Qatar Foundation Annual Research Conference. No. 2013. 2013.
- Porter, Brenda E., and Catherine Jacobson. "Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy." Epilepsy & Behavior 29.3 (2013): 574-577.
- Russo, Ethan, and Geoffrey W. Guy. "A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol." Medical hypotheses2 (2006): 234-246.
- Sarne, Yosef, et al. "The dual neuroprotective–neurotoxic profile of cannabinoid drugs." British journal of pharmacology 163.7 (2011): 1391-1401.
- Siniscalco, Dario, et al. "Cannabinoid receptor type 2, but not type 1, is upregulated in peripheral blood mononuclear cells of children affected by autistic disorders." Journal of autism and developmental disorders 43.11 (2013): 26862695.
- Zhang Longhua, Alger Bradley E., et al. “Enhanced Endocannabinoid Signaling Elevates Neuronal Excitability in Fragile X Syndrome” 2010
Brain Dev. 2013 Feb;35(2):146-54. doi: 10.1016/j.braindev.2012.03.011. Epub 2012 Apr 23.
Non-protein-bound iron and 4-hydroxynonenal protein adducts in classic autism.
Source
Department of Pathophysiology, Experimental Medicine & Public Health, University of Siena, Viale M. Bracci 16, Siena, Italy.
Abstract
A link between oxidative stress and autism spectrum disorders (ASDs) remains controversial with opposing views on its role in the pathogenesis of the disease. We investigated for the first time the levels of non-protein-bound iron (NPBI), a pro-oxidant factor, and 4-hydroxynonenal protein adducts (4-HNE PAs), as a marker of lipid peroxidation-induced protein damage, in classic autism. Patients with classic autism (n=20, mean age 12.0±6.2years) and healthy controls (n=18, mean age 11.7±6.5years) were examined. Intraerythrocyte and plasma NPBI were measured by high performance liquid chromatography (HPLC), and 4-HNE PAs in erythrocyte membranes and plasma were detected by Western blotting. The antioxidant defences were evaluated as erythrocyte glutathione (GSH) levels using a spectrophotometric assay. Intraerythrocyte and plasma NPBI levels were significantly increased (1.98- and 3.56-folds) in autistic patients, as compared to controls (p=0.0019 and p<0.0001, respectively); likewise, 4-HNE PAs were significantly higher in erythrocyte membranes and in plasma (1.58- and 1.6-folds, respectively) from autistic patients than controls (p=0.0043 and p=0.0001, respectively). Erythrocyte GSH was slightly decreased (-10.34%) in patients compared to controls (p=0.0215). Our findings indicate an impairment of the redox status in classic autism patients, with a consequent imbalance between oxidative stress and antioxidant defences. Increased levels of NPBI could contribute to lipid peroxidation and, consequently, to increased plasma and erythrocyte membranes 4-HNE PAs thus amplifying the oxidative damage, potentially contributing to the autistic phenotype.
Copyright © 2012 The Japanese Society of Child Neurology. Published by Elsevier B.V. All rights reserved.
Christian Bogner, M.D is fighting to show the world the benefits of Medical Cannabis for the Autism Spectrum. He is also the author of “The Endocannabinoid System as it Relates to Autism” and Medical Jane
Christian Bogner, M.D is fighting to show the world the benefits of Medical Cannabis for the Autism Spectrum. He is also the author of “The Endocannabinoid System as it Relates to Autism” His interest lies in strain research, specifically pioneering correct ratios for the wide spectrum of ASD.
Dr. Bogner, along with his Detroit based attorney Michael Komorn and researcher Joe Stone are fighting to add Autism as a qualifying condition for the medical Cannabis program in Michigan. The vote is July 20th. A yes vote would be the first in the country and potentially revolutionize the way we look at Autism.
His work is actively advocated by Lester Grinspoon, MD and many other experts in the field of neuropsychiatric research.
Follow him on Facebook at “Let it grow for Autism”.
Additional Research Showing How Medical Cannabis Benefits ASD
- Consequences of cannabinoid and monoaminergic system disruption in a mouse model of autism spectrum disorders [http://www.ncbi.nlm.nih.gov/pubmed/21886592]
Rules, Regulations, & Policy Solution For The Inclusion Of A New Medical Condition: Autism Spectrum Disorder
The approval of this Petition: Requesting The Inclusion Of A New Medical Condition: Autism Spectrum Disorder, that is being provided to the state Department of Health Medical Cannabis Program, so the advisory board can review and recommend to the department for approval of additional debilitating medical conditions that would benefit from the medical use of cannabis with the Lynn and Erin Compassionate Use Act. The approval of this petition would bring the Department of Health in compliance with the intent of the law and uphold the spirit of the Lynn and Erin Compassionate Use Act, 2007. Fulfilling both;“ Section 2. PURPOSE OF ACT.--The purpose of the Lynn and Erin Compassionate Use Act is to allow the beneficial use of medical cannabis in a regulated system for alleviating symptoms caused by debilitating medical conditions and their medical treatments” And Section 6. ADVISORY BOARD CREATED--DUTIES: The advisory board shall: A. review and recommend to the department for approval additional debilitating medical conditions that would benefit from the medical use of cannabis.” New Mexico’s medical cannabis history started in 1978. After public hearings the legislature enacted H.B. 329, the nation’s first law recognizing the medical value of cannabis...the first law.
Appendix A:
WHEREAS cannabis (marijuana) has been used as a medicine for at least 5,000 years and can be effective for serious medical conditions for which conventional medications fail to provide relief;
WHEREAS modern medical research has shown that cannabis can slow the progression of such serious diseases as Alzheimer’s and Parkinson’s and stop HIV and cancer cells from spreading; has both anti-inflammatory and pain-relieving properties; can alleviate the symptoms of epilepsy, PTSD and multiple sclerosis; is useful in the treatment of depression, anxiety and other mental disorders; and can help reverse neurological damage from brain injuries and stroke;
WHEREAS the World Health Organization has acknowledged the therapeutic effects of cannabinoids, the primary active compounds found in cannabis, including as an anti-depressant, appetite stimulant, anticonvulsant and anti-spasmodic, and identified cannabinoids as beneficial in the treatment of asthma, glaucoma, and nausea and vomiting related to illnesses such as cancer and AIDS;
WHEREAS the American Medical Association has called for the review of the classification of cannabis as a Schedule I controlled substance to allow for clinical research and the development of cannabinoid-based medicines;
WHEREAS the National Cancer Institute has concluded that cannabis has antiemetic effects and is beneficial for appetite stimulation, pain relief, and improved sleep among cancer patients;
WHEREAS the American Herbal Pharmacopoeia and the American Herbal Products Association have developed qualitative standards for the use of cannabis as a botanical medicine;
WHEREAS the U.S. Supreme Court has long noted that states may operate as “laboratories of democracy” in the development of innovative public policies;
WHEREAS twenty-eight states and the District of Columbia have enacted laws that allow for the medical use of cannabis;
WHEREAS seventeen additional states have enacted laws authorizing the medical use of therapeutic compounds extracted from the cannabis plant;
WHEREAS more than 17 years of state-level experimentation provides a guide for state and federal law and policy related to the medical use of cannabis;
WHEREAS accredited educational curricula concerning the medical use of cannabis have been established that meets Continuing Medical Education requirements for practicing physicians;
WHEREAS Congress has prohibited the federal Department of Justice from using funds to interfere with and prosecute those acting in compliance with their state medical cannabis laws, and the Department of Justice has issued guidance to U.S. Attorneys indicating that enforcement of the Controlled Substances Act is not a priority when individual patients and their care providers are in compliance with state law, and that federal prosecutors should defer to state and local enforcement so long as a viable state regulatory scheme is in place.
WHEREAS modern medical research has shown that cannabis can slow the progression of such serious diseases as Alzheimer’s and Parkinson’s and stop HIV and cancer cells from spreading; has both anti-inflammatory and pain-relieving properties; can alleviate the symptoms of epilepsy, PTSD and multiple sclerosis; is useful in the treatment of depression, anxiety and other mental disorders; and can help reverse neurological damage from brain injuries and stroke;
WHEREAS the World Health Organization has acknowledged the therapeutic effects of cannabinoids, the primary active compounds found in cannabis, including as an anti-depressant, appetite stimulant, anticonvulsant and anti-spasmodic, and identified cannabinoids as beneficial in the treatment of asthma, glaucoma, and nausea and vomiting related to illnesses such as cancer and AIDS;
WHEREAS the American Medical Association has called for the review of the classification of cannabis as a Schedule I controlled substance to allow for clinical research and the development of cannabinoid-based medicines;
WHEREAS the National Cancer Institute has concluded that cannabis has antiemetic effects and is beneficial for appetite stimulation, pain relief, and improved sleep among cancer patients;
WHEREAS the American Herbal Pharmacopoeia and the American Herbal Products Association have developed qualitative standards for the use of cannabis as a botanical medicine;
WHEREAS the U.S. Supreme Court has long noted that states may operate as “laboratories of democracy” in the development of innovative public policies;
WHEREAS twenty-eight states and the District of Columbia have enacted laws that allow for the medical use of cannabis;
WHEREAS seventeen additional states have enacted laws authorizing the medical use of therapeutic compounds extracted from the cannabis plant;
WHEREAS more than 17 years of state-level experimentation provides a guide for state and federal law and policy related to the medical use of cannabis;
WHEREAS accredited educational curricula concerning the medical use of cannabis have been established that meets Continuing Medical Education requirements for practicing physicians;
WHEREAS Congress has prohibited the federal Department of Justice from using funds to interfere with and prosecute those acting in compliance with their state medical cannabis laws, and the Department of Justice has issued guidance to U.S. Attorneys indicating that enforcement of the Controlled Substances Act is not a priority when individual patients and their care providers are in compliance with state law, and that federal prosecutors should defer to state and local enforcement so long as a viable state regulatory scheme is in place.
Lynn & Erin Compassionate Use Act Patient’s Coalition of New Mexico ~ A GrassRoots Movement!
UNITE-NETWORK-GROW-INFORM-KNOW-EDUCATE-ACTIVISM-VOTE-HEALTH-WELLNESS
(All Rights Reserved 04/20/2016)

No comments:
Post a Comment